研究概要:有機化学を礎に,新原理・新物質・新機能を開拓する
Research Summary : Organic chemistry to Explore New Principles, Reactions, and Fuctions
元素や分子,またその集合体機能を理解し,自在に制御することは化学者の夢です.当研究室では,電子顕微鏡によって分子を直接観察するという我々独自の手法によって分子の構造と反応性を解明する一方,鉄などのありふれた元素を用いた有機合成を有機デバイスの開発に展開することで「人々の夢や健やかな生活の実現」,「資源やエネルギー問題の解決」に貢献しています.
Chemists wish to understand and control the properties of elements, molecules, and their assemblies. Toward this goal, we study the structure and the reactivity of molecules by making full use of electron microscopy (SMART-EM; single-molecule Atomi-resolution time-resolved electron microscopy), and develop organic synthesis by the use for iron and fabricate organic electronic devices thereof.
研究内容
百聞は一見にしかず:「映像分子科学」の新しい世界
Seeing is believing - “Cinematic Chemistry” Explored
2007年,我々は有機分子の立体配座変換をリアルタイムで映像記録することに世界で初めて成功し,「百聞は一見にしかず」を実現しました (文献1).我々はこの方法をSMART-EM(Single-molecule atomic-resolution time-resolved electron microscopy)と名付けました.図1aがその画像です.最近は,抗生物質ダプトマイシンの生理活性発現の機構を解明し(図1b,文献2),化学反応における短寿命で微小な中間体を直接目で見てその反応機構を決定することもできるようになりました(文献3).世界最先端の高速高分解能電子顕微鏡(図1c)を駆使して実現した成果です.
Watching the movements of individual small molecules with our own eyes – a dream of people for many years. In 2007, we realized this dream by the cinematographic recording of the conformational changes of organic molecules in situ (Ref. 1) and named this method SMART-EM (see Figure 1a). Most recently, we elucidated the mechanism of bioactivity of the antibiotic daptomycin (Fig. 1b, Ref. 2), saw short-lived, microscopic intermediates in chemical reactions, and determined their reaction mechanisms (Ref. 3). The world most advanced electron microscope installed in the hospital campus next to the chemistry building (Fig. 1c) has played a pivotal role in this research.
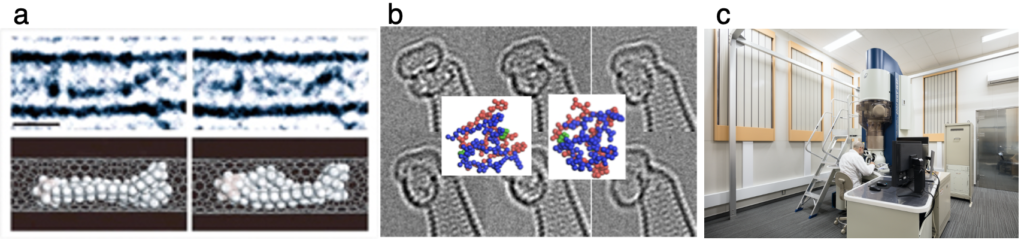
daptomycin dimer. c. Electron microscope in the Molecular Life Innovation Building.
図2には,NaとClのイオン対が順次集まって小さな結晶を形成する様子を記録した映像を示します.(文献4;https://youtu.be/Xo1yTnJo8Zs).この映像は「第63回科学技術映像祭研究開発部門優秀賞」を受賞しています.最先端科学と科学教育を結びつける「映像分子科学」という新しい分野の開拓を目指しています(文献5).
SMART-EM reveals the dynamic world of atoms and molecules that people have never seen before. For example, a video of the formation of a tiny crystal of NaCl attracted the attention of people worldwide, from elementary school kids to the general public (Figure 2, Ref. 4; https://youtu.be/Xo1yTnJo8Zs). A new field of “cinematic molecular science” is opening up (Ref. 5)
.
Reference
- Imaging single Molecules in Motion, M. Koshino, T. Tanaka, N. Solin, K. Suenaga, H. Isobe, E. Nakamura, Science 2007, 316, 853.
- Time-resolved Atomistic Imaging and Statistical Analysis of Daptomycin Oligomers with and without Calcium Ion. T. Nakamuro, K. Kamei, K. Sun, J. W. Bode, K. Harano, E. Nakamura, J. Am. Chem. Soc. https://doi.org/10.1021/jacs.2c03949
- Ionization and electron excitation of fullerene molecules in a carbon nanotube. A variable temperature/voltage transmission electron microscopic study, D. Liu, S. Kowashi, T. Nakamuro, D. Lungerich, K. Yamanouchi, K. Harano, E. Nakamura, Proc. Natl. Acad. Sci. USA.,2022, 119, e2200290119.
- Capturing the Moment of Emergence of Crystal Nucleus from Disorder, T. Nakamuro, M. Sakakibara, H. Nada, K. Harano, E. Nakamura, J. Am. Chem. Soc., 2021, 143, 1763.
- A Scientist and a Musician, E. Keinan, AsiaChem. 2021, 2, 96.
希少資源に頼らない有機合成:元素戦略
Iron-catalyzed Organic Synthesis:Element Strategy
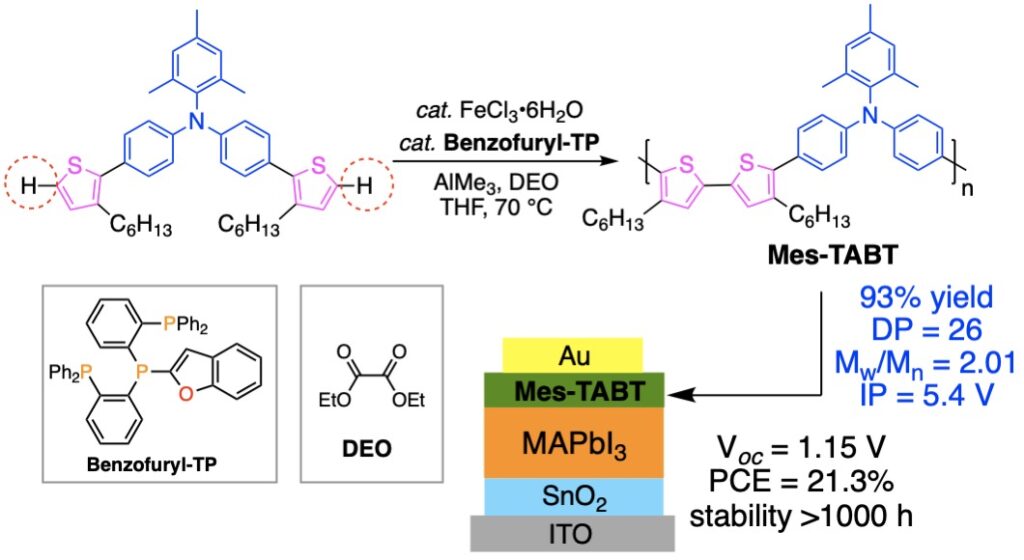
「元素戦略」は我々が2004年に提唱した日本の科学技術政策です.この考え方に基づき,我々は1990年代から「ありふれた元素」である鉄を触媒とする有機合成手法の開発で世界の先端を走ってきました.縮重した3d軌道に起因する多様なスピンおよび酸化還元特性をもつ鉄を触媒に用いてこれまでにない高活性な触媒系を実現,高効率・高選択的なC-H結合活性化反応を開発しました(文献1).図3にチオフェンのC−H活性化によって合成したポリマーと(文献2),これを正孔輸送材料として活用した高効率で長寿命のペロブスカイト太陽電池の例を示します(文献3).
Since the 1990s, we have been at the forefront of the development of organic synthesis using iron, a “ubiquitous element,” as a catalyst. However, iron is more difficult to control as a catalyst than palladium because of its diverse spin and redox properties due to the degenerate 3d orbitals. We have developed an active catalytic system by taking advantage of the small redox potential between Fe(III)/Fe(I) and developed an efficient and selective C-H bond activation reaction (Ref. 1). Figure 3 shows an example of a polymer synthesized by C-H activation of thiophene (Ref. 2) and an efficient and long-lived perovskite solar cells that utilize the polymer as a hole transport material (Ref. 3).
Reference
- Homocoupling-free iron-catalysed twofold C–H activation/cross-coupling of aromatics via transient connection of reactants, T. Doba, T. Matsubara, L. Ilies, R. Shang, E. Nakamura, Nat. Catal., 2019, 2, 400.
- Iron-Catalysed Regioselective Thienyl C–H/C–H Coupling, T. Doba, L. Ilies, W. Sato, R. Shang, E. Nakamura, Nat. Catal., 2021, 4, 631.
- Triarylamine/Bithiophene Copolymer with Enhanced Quinoidal Character as Hole-Transporting Material for Perovskite Solar Cells. H.-S. Lin, T. Doba, W. Sato, Y. Matsuo, R. Shang, E. Nakamura, Angew. Chem. Int. Ed. 2021, e202203949.
有機合成を基軸とした有機エレクトロニクス材料開発:エネルギー問題解決への貢献
Organic Electronics Materials Based on Iron-catalysis
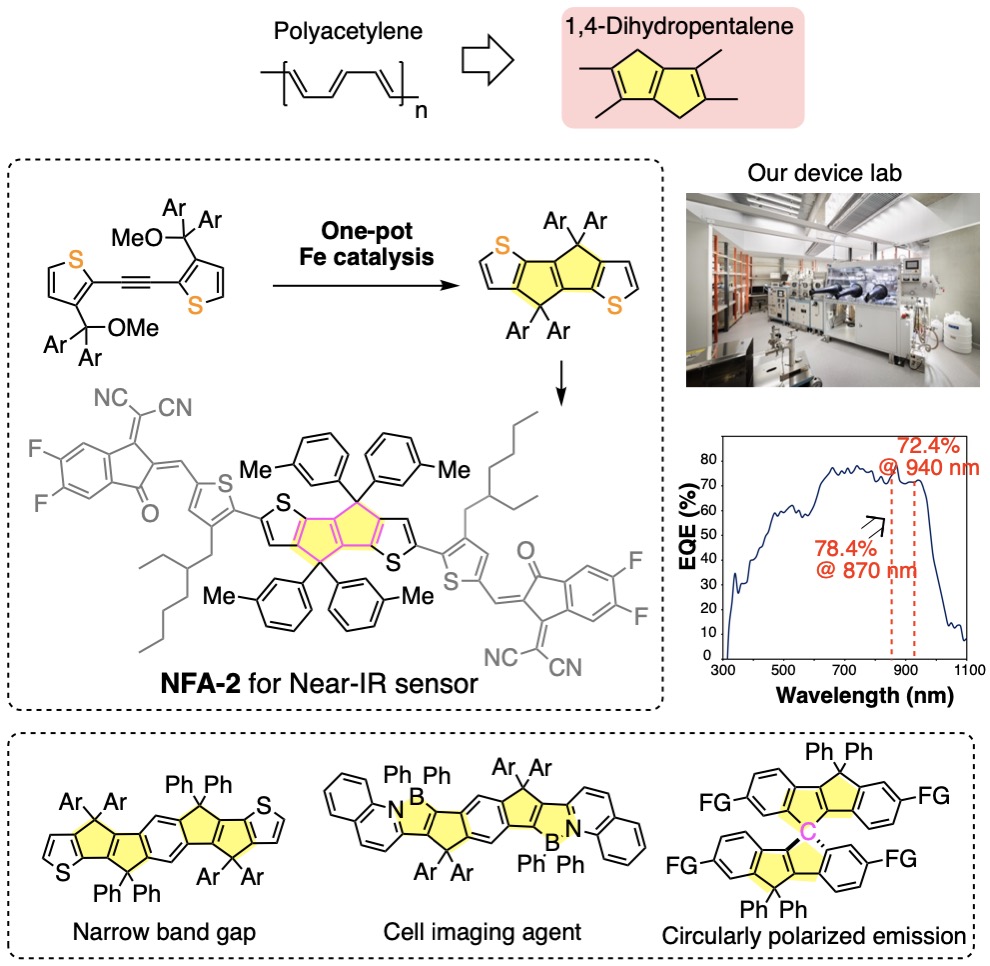
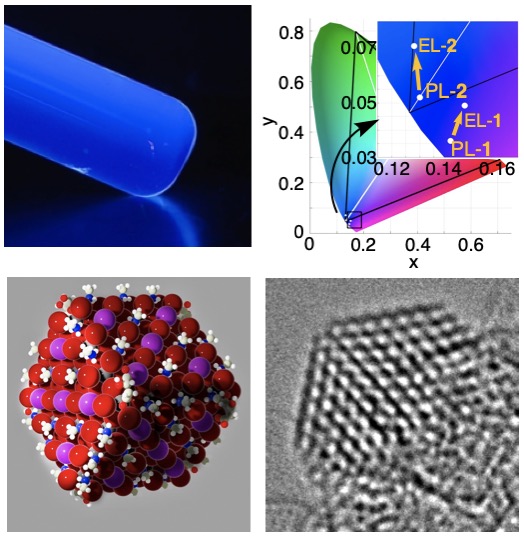
我々は有機合成の立場から,有機エレクトロニクスデバイスの研究を行っています(図4).有機伝導体の基本骨格であるポリアセチレンは構造がしなやかすぎて不安定ですが,1,4-ジヒドロペンタレンを組み込むことにより安定化することができます.この骨格を高活性の鉄触媒を使って短段階で合成し(文献1),高効率の近赤外検知器を作ることができました.さらにホウ素と窒素を持つ細胞イメージング試薬(文献2)や円偏光発光特性を示すスピロ分子を報告しました(文献3).青色発光量子ドットとLEDデバイス設計(図5,文献4),有機フラーレンを活用した機能性分子膜の開発(文献5),リチウムイオン電池の効率化に向けた分子設計にも取り組んでいます.
We also work on organic electronics devices based on our synthetic expertise. Polyacetylene is too flexible and unstable but can be stabilized by incorporating it in a planar 1,4-dihydropentalene skeleton. Thus, we prepared various planar conjugated molecules using highly active iron catalysts (Ref. 1). We synthesized a molecule that absorbs strongly in the near-infrared region and fabricated an efficient near-infrared detector. Using this design concept, we synthesized a cell imaging reagent with boron and nitrogen doping (Ref. 2) and spiro molecules with good circularly polarized emission properties (Ref. 3). We very recently prepared blue light-emitting quantum dots for LED devices (Ref. 4), ultra-thin molecular films utilizing the self-assembly of organic fullerenes (Ref. 5), and the materials for efficient lithium-ion batteries.
Reference
- Iron-Catalyzed Tandem Cyclization of Diarylacetylene to a Strained 1,4-Dihydropentalene Framework for Narrow-Band-Gap Materials, M. Chen, W. Sato, R. Shang, E. Nakamura, J. Am. Chem. Soc., 2021,143, 6323.
- B/N-Doped p-Arylenevinylene Chromophores: Synthesis, Properties , and Microcrystal Electron Crystallographic Study, H. Lu, T. Nakamuro, K. Yamashita, H. Yanagisawa, O. Nureki, M. Kikkawa, H. Gao, J. Tian, R. Shang, E. Nakamura, J. Am. Chem. Soc. 2020, 142, 18990.
- Axially Chiral Spiro-conjugated Carbon-bridged p-Phenylenevinylene Congeners: Synthetic Design and Materials Properties, H. Hamada, Y. Itabashi, R. Shang, E. Nakamura, J. Am. Chem. Soc., 2020, 142, 2059.
- De Novo Synthesis of Free-Standing Flexible 2D Intercalated Nanofilm Uniform over Tens of cm2, P. Ravat, H. Uchida, R. Sekine, K. Kamei, A. Yamamoto, O. Konovalov, M. Tanaka, T. Yamada, K. Harano, E. Nakamura, Adv. Mater. 2021, 34, 2146465.
- Precision synthesis and atomistic analysis of deep blue cubic quantum dots made via self-organization. O. J. G. L. Chevalier, T. Nakamuro, W. Sato, S. Miyashita, T. Chiba, J. Kido, R. Shang, E. Nakamura, J. Am. Chem. Soc., 2022, 144, 21146.
「革新分子技術」総括寄付講座について
本総括寄付講座は,東和薬品株式会社,日本電子株式会社,三菱ケミカル株式会社,株式会社地球快適化インスティテュート,住友化学株式会社のご寄付により設置された部局横断型の全学的な寄付講座です.以上に述べた研究を通して分子技術を革新し,資源利用の低エネルギー化,太陽光ならびにユビキタス元素の有効利用の道筋や医療イノベーション創出を確立していきます.また,ライフ・エネルギー分子技術イノベーションを実践できる国際的な若手人材の育成を行います.
